Getting the Kinks Out
The End of Partially Hydrogenated Oils
By Shawn Lee, Scientist
Right now, there’s a lot missing in grocery stores: toilet paper, bottled water, hand sanitizer, and… partially hydrogenated oils (PHOs). PHOs are popular with manufacturers of processed foods in need of a cheap butter alternative, or as preservatives to extend the shelf life of foods. However, since January 1, 2020, in the US you can no longer sell any foodstuff with PHOs as a major ingredient.
I became interested in food chemistry back in high school, where I investigated Kosher/Halal anti-foaming agents for maple syrup manufacturing in western Massachusetts. Niche as that was, I learned that, when dealing with commercial products, a technical analysis is always matched with an economic analysis, especially when an industry is seeking change. This realization led me to New Energy Risk, where I support the technoeconomic due diligence of our clients and pipeline. The types of analyses I did in the food industry were very similar to the energy technology analyses I work on at New Energy Risk.
Our clients need to stay abreast of industry regulations, which can immediately affect their feedstocks, off takers, and/or paths to commercialization. So it’s important for us to monitor old and new regulations, and to consider case studies that teach us how the businesses we work with might need to adapt, both commercially and technologically. Here’s one such example of policy change driving new technology implementation (or rather, old technology in new places); in this post, I’ll explain PHOs, how industry delayed their ban, and which replacement ingredients will be used going forward. It’s an interesting tale with lessons that spread beyond the breadbasket.
The Chemistry Behind Buttery Spreads
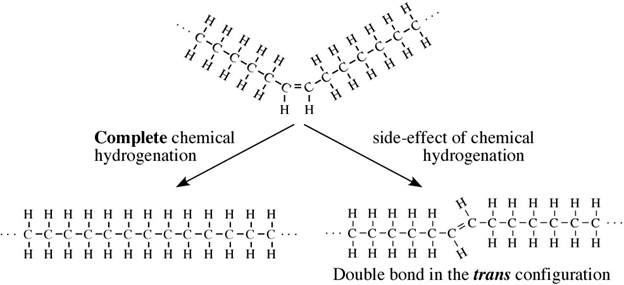
Partial hydrogenation is one of the most popular ways to control the freezing point of an oil, so it is spreadable like margarine: not too solid, but not too liquid either.
All oils, including vegetable oils, have three fatty acids linked to a glycerol, a simple compound that is colorless and odorless. These fatty acids are mostly long chains of carbon, which if fully saturated with hydrogen (i.e. lacking double bonds), can lay down flat on each other so that they are “unkinked.” This shape allows these fully hydrogenated fats to become solid at a lower temperature. Case in point, butter is fully hydrogenated, and at room temperature, butter is solid. But vegetable oil is not fully hydrogenated, so it appears “kinked,” and at room temperature, vegetable oil is liquid.
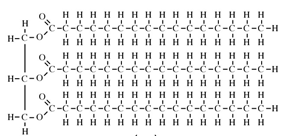 Butter: Solid, Unkinked http://www.indiana.edu/~oso/Fat/trans.html
Butter: Solid, Unkinked http://www.indiana.edu/~oso/Fat/trans.html
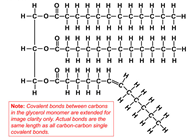 Vegetable Oil: Liquid, Kinked https://dlc.dcccd.edu/biology1-3/lipids
Vegetable Oil: Liquid, Kinked https://dlc.dcccd.edu/biology1-3/lipids
Most vegetable oils have kinks: double bonds that change the direction of the chain, keeping the oil molecules from stacking nicely and making them liquid at room temperature. To make them solid, you can add enough hydrogen to straighten out all those kinks, but that would result in a solid block of fat. Instead, food manufacturers add less hydrogen to do a partial hydrogenation, which straightens out only some of those kinks, resulting in a consistently spreadable product, or a PHO.
Unfortunately, in real life nothing is perfect, and in practice, chemical partial hydrogenation turns some of an oil’s double bonds from “cis” to “trans,” which are different molecular formations. You may have heard of trans fatty acids or “trans fats” before; these are PHOs with slightly crooked double bonds.
Double bond in the cis configuration:
Regulation and Industry
These trans fats have been correlated with increased LDL cholesterol, which has in turn been correlated with heart disease, the leading cause of death in the US according to the Center for Disease Control (in the times before COVID-19, anyway). Once these correlations were discovered, food regulations could mandate an end to the use of trans fats (and PHOs by extension) to avoid a further public health crisis. The US Food and Drug Administration (FDA) finally began taking action in 2015, concluding that PHOs were no longer Generally Recognized as Safe (GRAS).
But then on June 17, 2015, Industry complained about the regulations. Some claimed that PHOs are safe as minor constituents in coloring and flavoring additives, baking spray, and pie pan linings. The Grocery Manufacturers Association (GMA) cited two studies that relied on a model of animal metabolism… even though human data were available. It argued that trans-fat exposure against mortality risk should be modeled on an S-shaped curve as opposed to a linear curve, so that at low concentrations there would be almost no health effect. Unfortunately, these studies had poor-quality data at low exposure rates, and in some cases counted data twice. The FDA decided that the GMA finding — that PHOs are not necessarily bad for you at low levels — was unsupported. After failing to provide an environmental review, and given several other technical deficiencies, the FDA decided that PHOs as minor additives will still be banned and the petition was denied in its entirety.
However, the FDA did give the GMA an extension to figure out what could serve as a replacement for minor constituent PHOs. That extension expired on June 18, 2019, the last day it was legal to purposefully add PHOs into any foodstuff made in the US. Manufacturers and retailers then had six months to get products with PHOs as a major constituent through the supply chain, but by now, those products should mostly be gone from shelves. Since Jan 1, 2020, those products can no longer be sold, though minor additive- containing products may legally remain on the US shelves until 2021.
Alternative Technologies
Fortunately for food manufacturers, there are two primary alternatives that give us most of the benefits of partial hydrogenation without the health risks.
Fractional distillation: Natural oils are a mixture of different molecules with different melting points. You can separate these molecules by boiling off the mixture bit by bit (since the molecules will boil off at different temperatures, in a similar order to their freezing points).Then you can tailor your mix of oils and fats so in the aggregate they are a mix of solid and liquid around room temperature. Combine that mix with a small amount of surfactant to keep them from re-separating, and you’ll have a healthier replacement for PHOs.
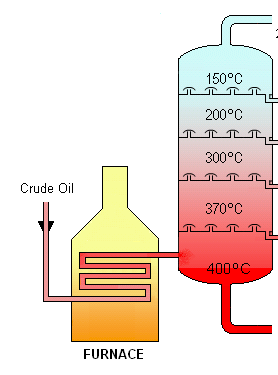 Fractional distillation, so you can separate the oils by boiling point, https://en.wikipedia.org/wiki/Fractional_distillation
Fractional distillation, so you can separate the oils by boiling point, https://en.wikipedia.org/wiki/Fractional_distillation
Interesterification: Alternatively, if the starting oil has lots of unsaturated fatty acids on one molecule, one can spread them more evenly amongst oil mixtures, so that fewer of the molecules are perfectly flat, and fewer are completely kinked. This will reduce the differences between the freezing points of the oil constituents, so they’ll be easier to keep from separating.
In renewable diesels, this same interesterification of vegetable oils with methyl acetate, a relatively small molecule, shrinks the average size of the molecules and decreases its viscosity, allowing it to be used in a conventional diesel engine.
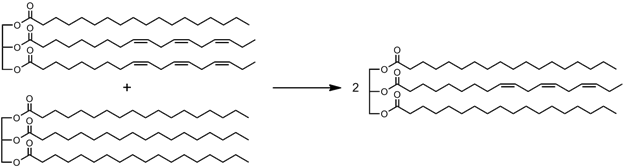 Redistributing the double bonds (kinks) more evenly through the molecules, https://en.wikipedia.org/wiki/Interesterified_fat
Redistributing the double bonds (kinks) more evenly through the molecules, https://en.wikipedia.org/wiki/Interesterified_fat
Industry on Board
These regulations have been phased in over time; thus far we’ve seen no major hiccups in the removal of major constituent PHOs from the food supply chain, and this regulation has helped spur a number of new PHO substitutes without trans fats. These additives tend to be tailored to their use while reducing the LDL levels associated with conventional PHOs. In the long term, we could see a reduction in heart attacks and strokes in the US.
This is an example of regulation encouraging industry to adopt new technologies for the greater good. These are innovation opportunities for food chemical manufacturers, and the industrial agricultural companies that supply the ingredients they need. In the long term, as potential health results are realized across the population, we’ll also observe if/how buttery spread capital markets shift and consumer demands change. Which reminds us that regulation is one way to grease the wheels of invention.
###